Cracking the Code of Alzheimer’s Diagnosis with Machine Learning

A Journey Through Data Science, Medicine, and the Unknown
Imagine a disease that slowly erodes everything that makes you who you are. A disease that creeps in unnoticed, until one day, the world no longer makes sense. Alzheimer’s Disease (AD) is precisely that—an irreversible neurodegenerative disorder that affects millions worldwide. In Switzerland alone, over 150,000 people suffer from dementia, with 60% of those cases attributed to AD. And yet, despite decades of research, no cure exists.
The problem? Alzheimer’s is typically diagnosed only when it's already too late—when cognitive decline has reached an irreversible stage.
What if we could see it coming earlier? What if we could predict it, long before symptoms become evident? That’s where this story begins. Over a semester at FHNW, with guidance by researchers from the University of Miami Miller School of Medicine, together with my team I worked on applying Machine Learning (ML) to Alzheimer’s diagnostics using biomarkers. The mission: to develop predictive models that can flag early warning signs of Alzheimer’s based on a multi-modal approach—combining gut bacteria, cerebrospinal fluid (CSF) biomarkers, sleep behavior, and MRI scans.
This made our project not just another ML exercise. It was a challenge that sat at the intersection of medicine and AI, a great blend of science, technology, and real-world impact.
The Challenge: Diagnosing the Undiagnosable
The fundamental challenge with Alzheimer’s is that it progresses silently. By the time it’s diagnosed, much of the brain’s neural infrastructure has already deteriorated. Current diagnostic methods rely on cognitive tests and brain imaging, but these are expensive, invasive, and often subjective.
We set out to explore whether changes in gut bacteria, sleep patterns, and cerebrospinal fluid biomarkers could serve as early indicators of cognitive decline leading to AD. And if that wasn’t ambitious enough, we also wanted to see whether combining all of these factors—alongside brain imaging—could improve diagnostic accuracy.
Key questions we asked:
- Can gut bacteria changes predict Alzheimer’s onset?
- Are cerebrospinal fluid biomarkers (β-Amyloid, Tau, P-Tau) effective early predictors?
- Can sleep behavior changes indicate cognitive decline?
- What happens when we merge these datasets and integrate MRI scans into the model?
The Data: Extracting Signals from Chaos
We worked with data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)—one of the most extensive datasets available for Alzheimer’s research. This dataset contained:
- Gut Bacteria Profiles – Over 100 metabolites measured over time
- CSF Biomarkers – β-Amyloid, Tau, and P-Tau levels
- Sleep Behavior – Derived from caregiver questionnaires (NPI scores)
- MRI Scans – 3D brain imaging with 1.5 Tesla scanners
- Longitudinal Diagnosis Data – Tracking patients as they transitioned from mild cognitive impairment (MCI) to AD
Each data source brought its own challenges, with missing values, irregular collection frequencies, and high dimensionality. We integrated them all into a meaningful, unified dataset.
The Approach: Merging Medicine with Machine Learning
Our strategy involved a multi-step data pipeline that systematically cleaned, merged, and prepared these datasets for training machine learning models.
Step 1: Data Preprocessing & Feature Engineering
- Handling Missing Values – We used forward and backward imputation for missing diagnoses and feature values.
- Feature Selection – Applied logistic regression, decision trees, and backward elimination to identify the most predictive biomarkers.
- Calculating Longitudinal Changes – Rather than using raw values, we focused on changes over time, calculating differences between visits.
- MRI Preprocessing – Applied skull-stripping to remove non-brain structures using SynthStrip.
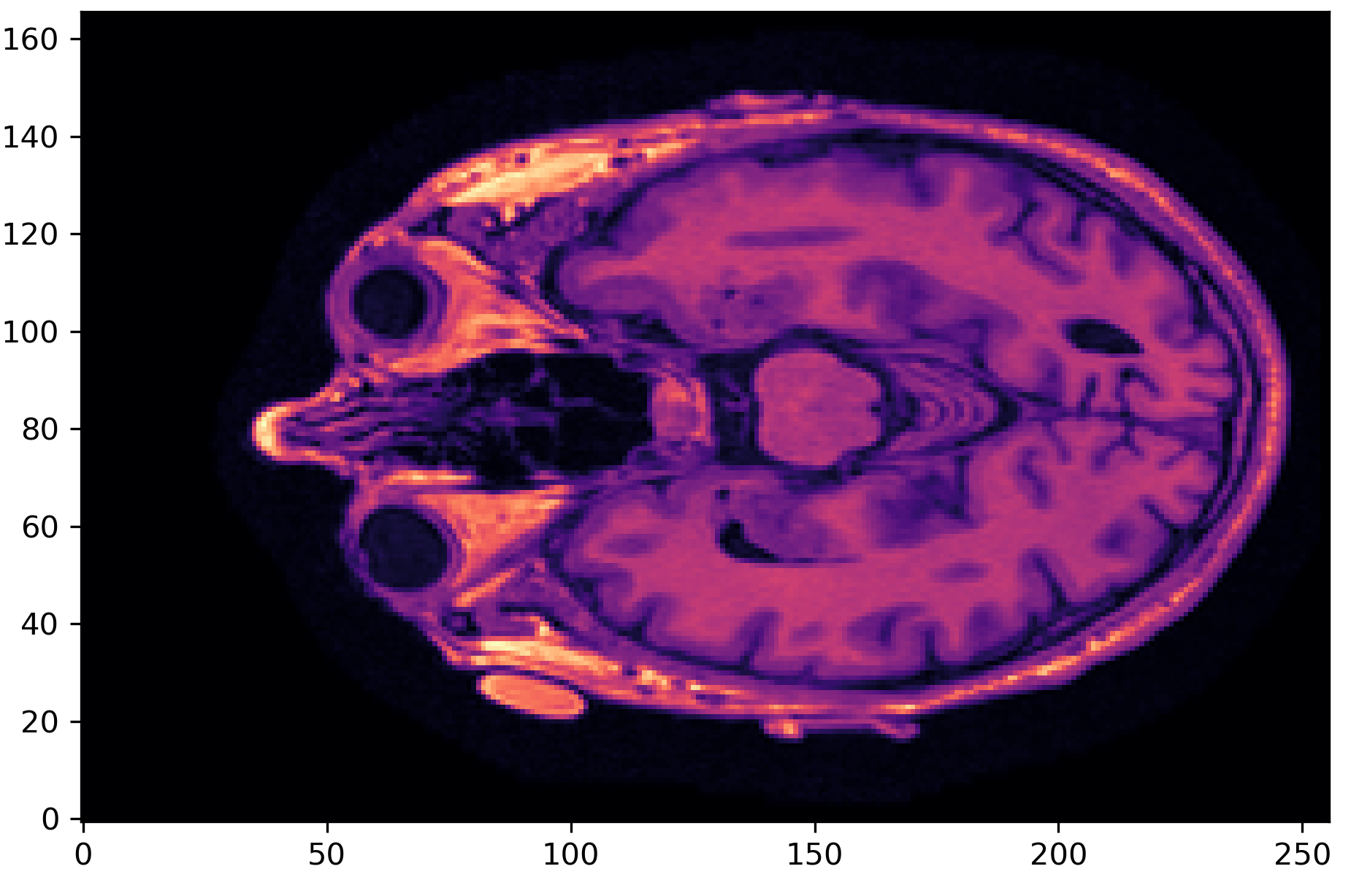
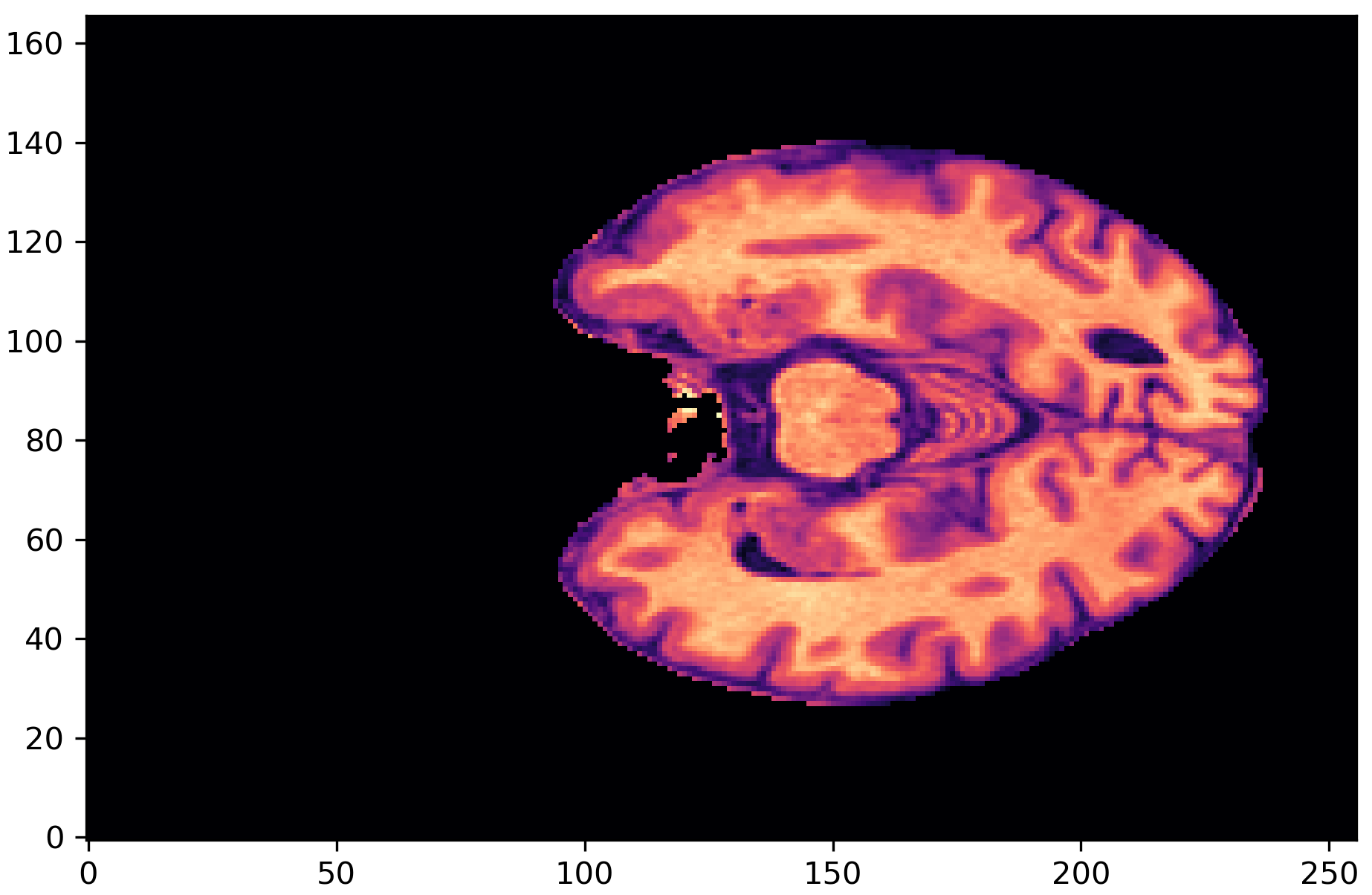
Step 2: Training Predictive Models
We trained four types of classifiers:
- Logistic Regression – Simple but interpretable
- Decision Trees – Captured non-linear relationships
- K-Nearest Neighbors (KNN) – Effective for structured data
- Multilayer Perceptron (MLP) – Captured complex feature interactions
Each model was trained on individual datasets (gut bacteria, CSF, sleep) as well as on combined datasets to explore whether integration improved predictive power.
Step 3: Multi-Modal Deep Learning (Combining Biomarkers with MRI)
We designed a multi-modal neural network that took tabular features from biomarkers and integrated them with MRI scans using a 3D Convolutional Neural Network (3D-CNN).

Our multi-modal model integrates tabular biomarker data with 3D-CNN-based MRI feature extraction, allowing the network to learn both structured and spatial dependencies in the patient data. By concatenating extracted image features with processed biomarker inputs, the model effectively combines quantitative biological markers with deep spatial representations of brain morphology, improving predictive performance over single-modal approaches.
The Results: What We Learned (and What We Didn’t)
Key Takeaways:
Sleep Data was the Strongest Predictor
- Sleep behavior changes achieved the highest individual prediction accuracy (82% precision).
- Suggests a strong link between sleep disturbances and Alzheimer’s progression.
Gut Bacteria & CSF Data Alone Weren’t Enough
- Predictions based on gut bacteria and CSF biomarkers were only moderately effective (~65% precision).
- These biomarkers are likely useful in combination with other factors, but not in isolation.
Multi-Modal Model Improved Accuracy
- Combining MRI with biomarker data increased precision to 72%.
- Suggests that integrating multiple data types leads to more reliable diagnoses
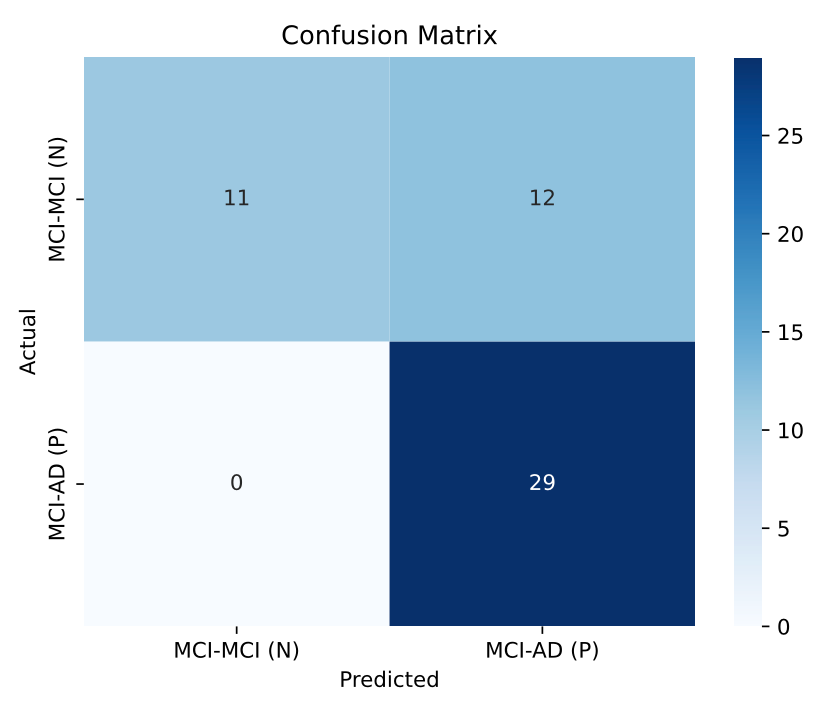
Single-Visit Predictions Remain a Challenge
- Our models performed best when analyzing changes across multiple visits, but struggled with single-visit predictions.
- Alzheimer’s progression is a slow process—biomarkers may need longer observation periods to be truly predictive.
The Bigger Picture: What This Means for Alzheimer’s Research
One thing is clear. Machine learning can assist in early Alzheimer’s detection, but it’s not a silver bullet. The challenge isn’t just in training better models—it’s in collecting better data. Reliable early diagnosis requires:
- Better Sleep Monitoring – More precise sleep tracking tools could improve prediction accuracy.
- More MRI Data – Larger datasets with higher resolution imaging would enhance deep learning models.
- Longitudinal Studies – More comprehensive, multi-year tracking of biomarkers is essential.
Our multi-modal approach showed promise, but it also revealed the limitations of current datasets. As AI in healthcare advances, combining multiple sources of patient data will be key to improving early Alzheimer’s diagnosis.
Final Thoughts
This was more than just a student project. It was a dive into one of the most pressing medical challenges of our time. It was a lesson in working across disciplines, in tackling real-world complexity, and in understanding the power (and limitations) of AI in medicine.
We didn’t find the magic solution. But we did uncover insights that could help push Alzheimer’s research forward. And in a field where every breakthrough counts, that’s a step worth taking.
Want to dive into the details? Check out the full IEEE paper draft and the GitHub repository where we documented our process and code.
I worked on this project in collaboration with Florin Barbisch and Patrick Schürmann, as part of our Bachelor studies in Data Science at FHNW.
This project challenge was supported by Prof. Dr. Arzu Çöltekin and Dr. Leticia Fernández Moguel from the FHNW University of Applied Sciences and Arts Northwestern Switzerland, in collaboration with Dr. Azizi Seixas and Dr. Alberto Ramos from the University of Miami Miller School of Medicine. Their combined expertise in data science, neuroimaging, and medical research laid the foundation for our work on predictive Alzheimer’s diagnostics, bridging the gap between AI and healthcare.
